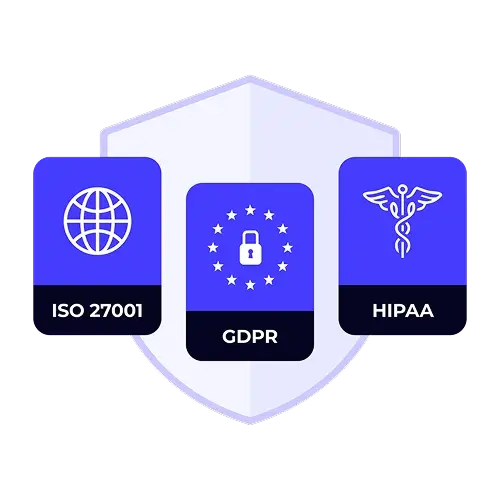
Speed up global drug and device launches with secure, validated translation management that meets FDA 21 CFR Part 11 and EU MDR standards. XTM's complete platform blends AI-powered translation with specialized tools for clinical trials, regulatory filings, and medical device localization.
With audit-ready tracking and enterprise-grade security, XTM Cloud ensures every document is accurate, compliant, and delivered on time—helping life sciences teams accelerate approvals and maintain patient trust.


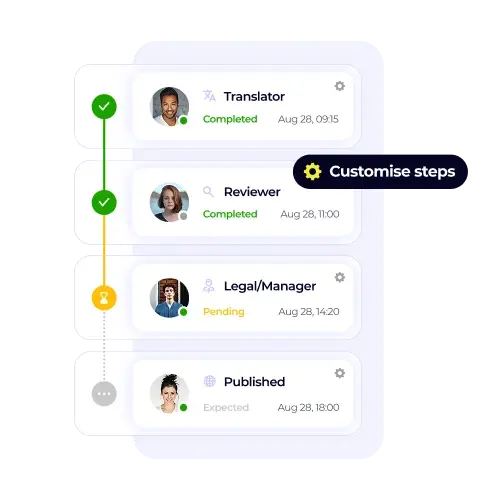
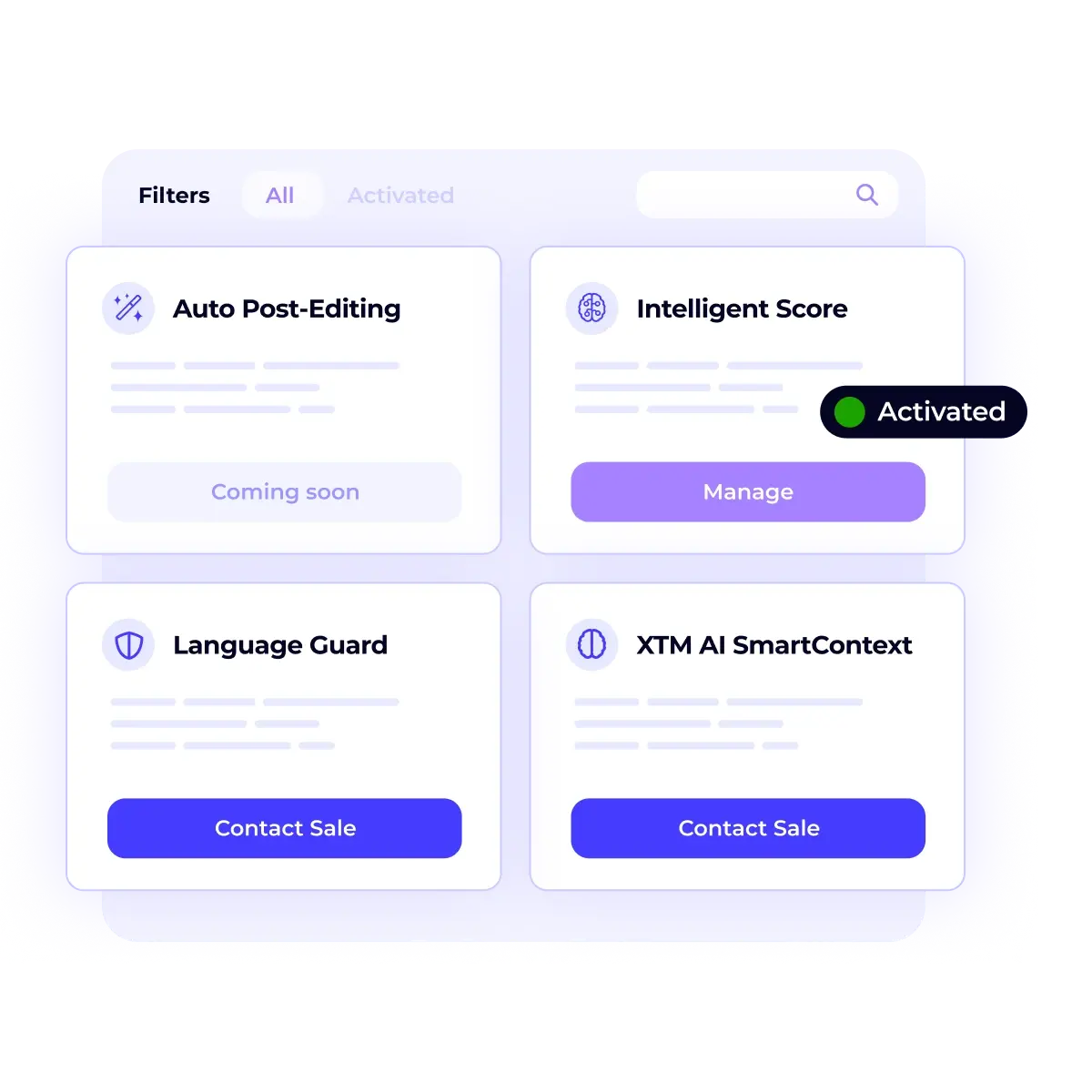
/USPs%20-%20Multi-format%20file%20support.webp)







.jpg)







.png?width=206&height=206&name=frame%20(19).png)






-2.png?width=280&height=280&name=Untitled%20design%20(4)-2.png)