Managing medical documentation across international markets requires precise language handling and strict regulatory adherence. Modern pharmaceutical companies face mounting pressure to deliver compliant translations that meet diverse regulatory standards while accelerating time-to-market for life-saving treatments.
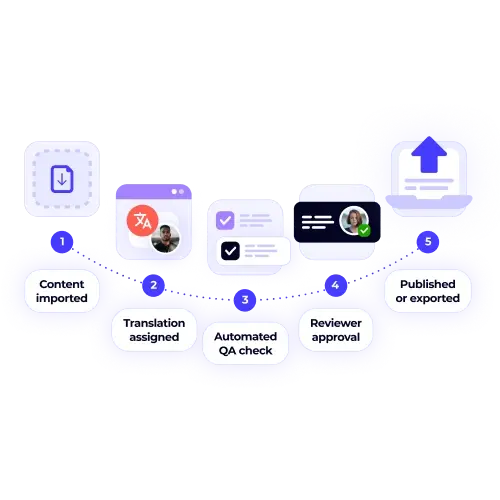
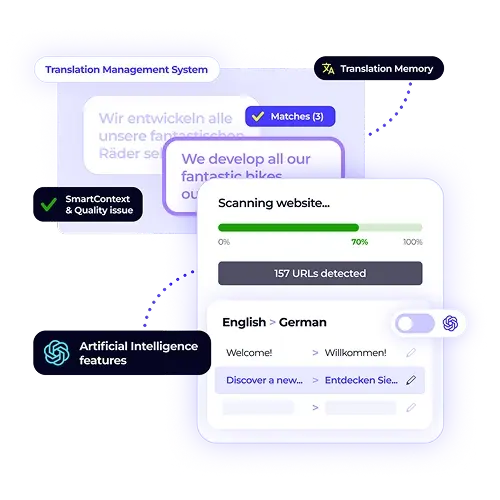
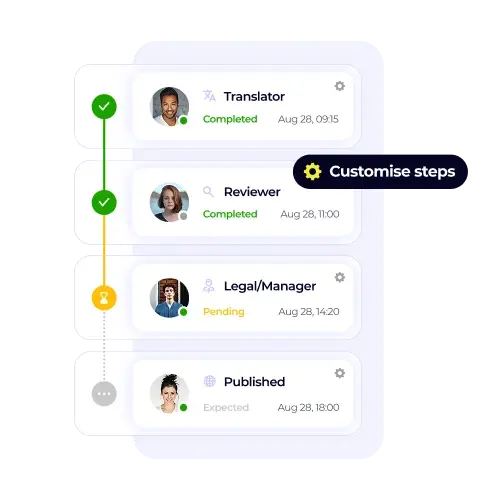
XTM provides pharmaceutical translation solutions that address these critical challenges through purpose-built workflows, automated quality controls, and comprehensive audit systems. Our platform helps pharmaceutical organisations maintain accuracy, ensure compliance, and reduce approval timelines across all global markets.










.jpg)







.png?width=206&height=206&name=frame%20(19).png)






-2.png?width=280&height=280&name=Untitled%20design%20(4)-2.png)